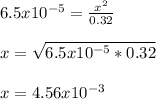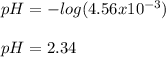Chemistry, 29.03.2021 22:00 underswap25
Benzoic acid, HC6H5CO2,is a monoprotic acid(only one H+ ionizes)with a Ka=6.5×10^-5. Calculate [H+] and the pH of a .32M solution of benzoic acid. PLEASE ANSWER.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1
You know the right answer?
Benzoic acid, HC6H5CO2,is a monoprotic acid(only one H+ ionizes)with a Ka=6.5×10^-5. Calculate [H+]...
Questions

Mathematics, 29.05.2021 05:40

Mathematics, 29.05.2021 05:40







English, 29.05.2021 05:40

Mathematics, 29.05.2021 05:40



Biology, 29.05.2021 05:40







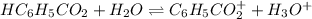
![[H_3O^+]=[H^+]](/tpl/images/1228/3574/877c7.png) , we can set up the equilibrium expression in terms of
, we can set up the equilibrium expression in terms of  (reaction extent) to obtain:
(reaction extent) to obtain:![Ka=\frac{[C_6H_5CO_2^-][H_3O^+]}{[HC_6H_5CO_2]} \\\\6.5x10^{-5}=\frac{x^2}{0.32-x}](/tpl/images/1228/3574/4456d.png)