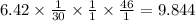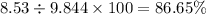Nitrogen monoxide is produced by combustion in an automobile engine. For the following reaction, 6.42 grams of nitrogen monoxide are mixed with excess oxygen gas . The reaction yields 8.53 grams of nitrogen dioxide . nitrogen monoxide ( g ) oxygen ( g ) nitrogen dioxide ( g ) What is the theoretical yield of nitrogen dioxide

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
Nitrogen monoxide is produced by combustion in an automobile engine. For the following reaction, 6.4...
Questions

Social Studies, 28.10.2020 01:50


Mathematics, 28.10.2020 01:50


Computers and Technology, 28.10.2020 01:50

Mathematics, 28.10.2020 01:50

Chemistry, 28.10.2020 01:50

Computers and Technology, 28.10.2020 01:50

History, 28.10.2020 01:50


English, 28.10.2020 01:50

English, 28.10.2020 01:50

Geography, 28.10.2020 01:50


Biology, 28.10.2020 01:50

Mathematics, 28.10.2020 01:50

Mathematics, 28.10.2020 01:50

History, 28.10.2020 01:50


Social Studies, 28.10.2020 01:50