
Chemistry, 29.03.2021 16:50 lindamillscotton90
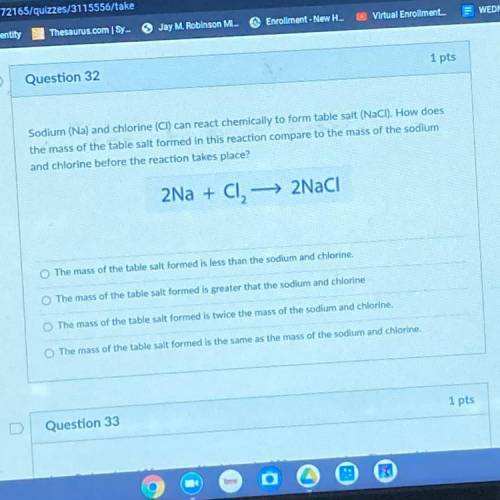
Sodium (Na) and chlorine (CI) can react chemically to form table salt (NaCl). How does the mass of the table salt formed in this reaction compare to the mass of the sodium and chlorine before the reaction takes place?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Write skeleton equations for the following reactions c. aluminum(s)+copper(i) chloride(aq) > aluminum chloride(aq)+copper(s)
Answers: 1


Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Sodium (Na) and chlorine (CI) can react chemically to form table salt (NaCl). How does
the mass of...
Questions

History, 29.10.2019 18:31



Mathematics, 29.10.2019 18:31

Arts, 29.10.2019 18:31



History, 29.10.2019 18:31


Mathematics, 29.10.2019 18:31


Advanced Placement (AP), 29.10.2019 18:31






Mathematics, 29.10.2019 18:31


History, 29.10.2019 18:31



