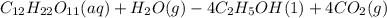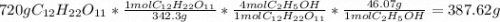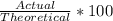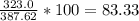6 minutes ago • Chemistry • High School
Ethanol (C2H5OH) is produced from the fermentation of
...

Chemistry, 29.03.2021 06:30 hoolio4495
6 minutes ago • Chemistry • High School
Ethanol (C2H5OH) is produced from the fermentation of
sucrose in the presence of enzymes.
AC
C12H22011(aq) + H2O(g) 4 C2H5OH(1) + 4 CO2(g)
Determine the theoretical yield and the percent yields of ethanol if 720.g
sucrose undergoes fermentation and 323.0 g ethanol is obtained.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
Questions




Chemistry, 10.06.2021 14:00



Mathematics, 10.06.2021 14:00

Mathematics, 10.06.2021 14:00

English, 10.06.2021 14:00

Health, 10.06.2021 14:00



Physics, 10.06.2021 14:00


Mathematics, 10.06.2021 14:00

Mathematics, 10.06.2021 14:00



Chemistry, 10.06.2021 14:00