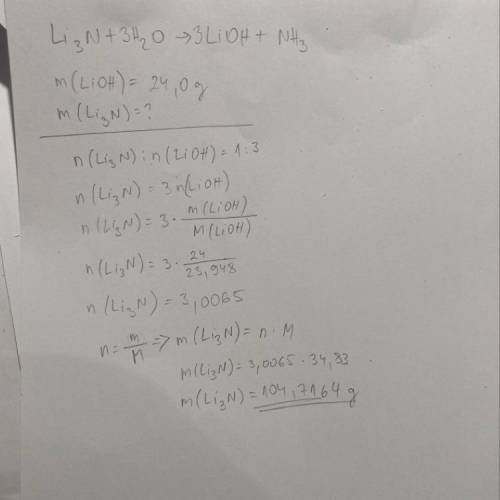Chemistry, 29.03.2021 02:10 makaylamsikahema
WILL GIVE BRANLIEST
1)how many liters of HCl are produced when 47.2 L of chlorine are reacted with excess hydrogen at STP
2)if you need to make 24.0 g LiOH , how many grams of Li3N must you react with excess water?
3)How many moles of hydrogen gas can be produced from 13.2 moles of hydrochloric acid (HCl)
4)if 34.9g of copper (ll) chloride reacts with 42.1g of sodium nitrate what is the limiting reactant?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
WILL GIVE BRANLIEST
1)how many liters of HCl are produced when 47.2 L of chlorine are reacted with...
Questions

Mathematics, 12.01.2022 07:30


Mathematics, 12.01.2022 07:30

Mathematics, 12.01.2022 07:30


Computers and Technology, 12.01.2022 07:40


Computers and Technology, 12.01.2022 07:40






Mathematics, 12.01.2022 07:40

Social Studies, 12.01.2022 07:40



Geography, 12.01.2022 07:40


Geography, 12.01.2022 07:40