

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Acid spills are often neutralized with sodium carbonate. For example
Na CO2 (s) + H2SO4 (aq) → Na2S...
Questions



Social Studies, 27.10.2021 08:50

Mathematics, 27.10.2021 08:50

History, 27.10.2021 08:50

Mathematics, 27.10.2021 08:50

History, 27.10.2021 08:50

Mathematics, 27.10.2021 08:50

English, 27.10.2021 08:50



Mathematics, 27.10.2021 08:50



History, 27.10.2021 08:50




Mathematics, 27.10.2021 08:50

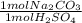 = 45 mol NaCO₂
= 45 mol NaCO₂

