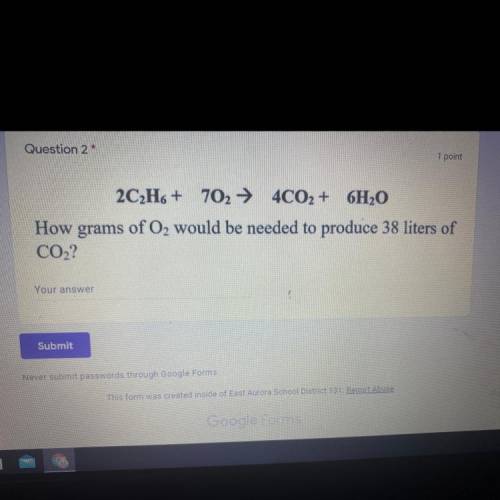
2C2H6 + 702 + 4CO2 + 6H2O
How grams of O2 would be needed to produce 38 liters of
CO2?
<...


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1

Chemistry, 23.06.2019 08:00
Which of the following notations would be the appropriate and final way to display the formula for magnesium chloride a. mgcl2 b. mg+2cl–1 c. mgcl2 d. mgcl
Answers: 2
You know the right answer?
Questions

Mathematics, 13.10.2019 22:30

Social Studies, 13.10.2019 22:30



Physics, 13.10.2019 22:30

Mathematics, 13.10.2019 22:30

Mathematics, 13.10.2019 22:30




English, 13.10.2019 22:30

English, 13.10.2019 22:30

Mathematics, 13.10.2019 22:30



Mathematics, 13.10.2019 22:30

Biology, 13.10.2019 22:30


History, 13.10.2019 22:30

English, 13.10.2019 22:30