
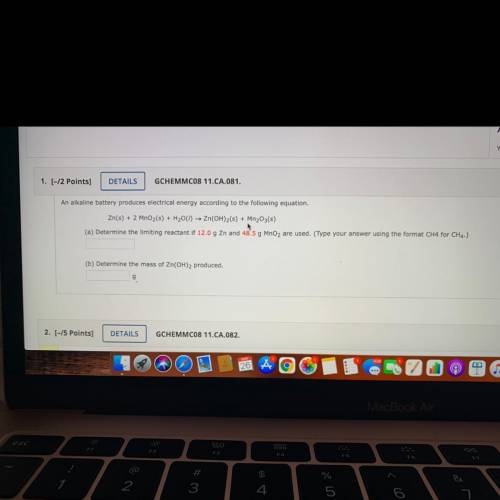
An alkaline battery produces electrical energy according to the following equation.
Zn(s) + 2 MnO2(s) + H2001) — Zn(OH)2(s) + Mn2O3(s)
(a) Determine the limiting reactant if 12.0 g Zn and 48.5 g MnO2 are used. (Type your answer using the format CH4 for CH4.)
(b) Determine the mass of Zn(OH)2 produced.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
An alkaline battery produces electrical energy according to the following equation.
Zn(s) + 2 MnO2(...
Questions

Chemistry, 19.09.2019 17:10



History, 19.09.2019 17:10



Physics, 19.09.2019 17:10
















