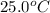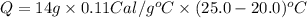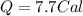Chemistry, 16.10.2019 15:30 oofoofoof1
Suppose a 14-gram sample of iron is heated from 20.0°c to 25.0°c. the specific heat of iron is 0.11 cal/g°c. how much heat energy was absorbed by the iron? 38.5 cal 7.7 cal 636 cal 69.3 cal

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Suppose a 14-gram sample of iron is heated from 20.0°c to 25.0°c. the specific heat of iron is 0.11...
Questions

Mathematics, 13.11.2020 19:30

Mathematics, 13.11.2020 19:30


Mathematics, 13.11.2020 19:30

English, 13.11.2020 19:30


History, 13.11.2020 19:30

English, 13.11.2020 19:30


Mathematics, 13.11.2020 19:30

History, 13.11.2020 19:30



Mathematics, 13.11.2020 19:30

Spanish, 13.11.2020 19:30

Social Studies, 13.11.2020 19:30

Business, 13.11.2020 19:30

Mathematics, 13.11.2020 19:30

Mathematics, 13.11.2020 19:30

Physics, 13.11.2020 19:30

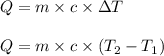
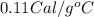
 = change in temperature
= change in temperature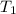 = initial temperature =
= initial temperature = 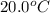
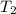 = final temperature =
= final temperature =