Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1



Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1
You know the right answer?
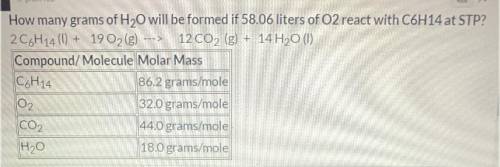
How many grams of H2O will be formed if 58.06 liters of O2 react with C6H14 at STP?
Balanced equati...
Questions

Mathematics, 06.11.2020 04:40



Mathematics, 06.11.2020 04:40



Mathematics, 06.11.2020 04:40



Mathematics, 06.11.2020 04:40


Mathematics, 06.11.2020 04:40

Mathematics, 06.11.2020 04:40

English, 06.11.2020 04:40



Mathematics, 06.11.2020 04:40

Mathematics, 06.11.2020 04:40

Computers and Technology, 06.11.2020 04:40