
Chemistry, 26.03.2021 03:50 haileyglowiak8183
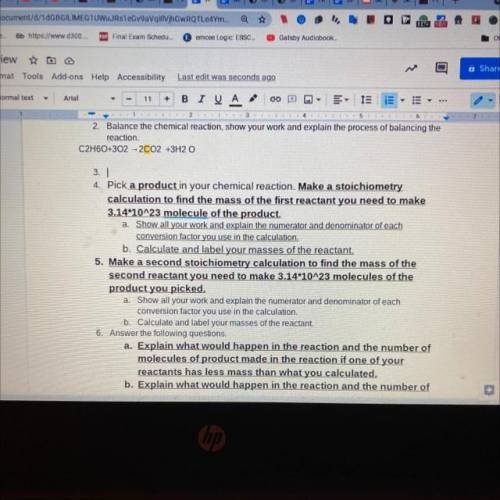
.Pick a product in your chemical reaction. Make a stoichiometry
calculation to find the mass of the first reactant you need to make
3.14*10^23 molecule of the product.
fonch

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3

You know the right answer?
.Pick a product in your chemical reaction. Make a stoichiometry
calculation to find the mass of the...
Questions


English, 11.11.2021 08:40

English, 11.11.2021 08:40

Mathematics, 11.11.2021 08:40



Law, 11.11.2021 08:40

History, 11.11.2021 08:40

English, 11.11.2021 08:40

English, 11.11.2021 08:40


Biology, 11.11.2021 08:40




Mathematics, 11.11.2021 08:40

Mathematics, 11.11.2021 08:40



Arts, 11.11.2021 08:40



