
Chemistry, 25.03.2021 22:40 heleneallie7ou1fnk
The temperature of water decreases on the dissolution of NH4NO3. From this observation, we can conclude that the solution process would be best described asa)exothermic with a positive entropy change. b)exothermic with a negative entropy change. c)endothermic with a negative entropy change. d)endothermic with a positive entropy change. e)exothermic with a negative free energy change

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
The temperature of water decreases on the dissolution of NH4NO3. From this observation, we can concl...
Questions



Chemistry, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00




Computers and Technology, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

History, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

History, 25.11.2020 01:00

Biology, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00


Social Studies, 25.11.2020 01:00


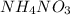 , it means heat has been absorbed by
, it means heat has been absorbed by 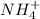 and
and 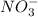 , randomness will increase and thus there is a positive entropy change.
, randomness will increase and thus there is a positive entropy change.

