The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
<...

Chemistry, 25.03.2021 22:10 iesps010411
The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
Ag+ (aq) + e^- -> Ag(s)
Al(s) -> Al^3+ (aq) + 3e^-
What is the coefficient of silver in the final, balanced equation for this reaction?
A. 1
B. 2
C. 3
D. 4
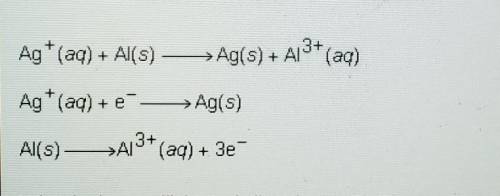

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Questions


English, 12.02.2021 23:30

Biology, 12.02.2021 23:30

Social Studies, 12.02.2021 23:30


Mathematics, 12.02.2021 23:30



Mathematics, 12.02.2021 23:30

Health, 12.02.2021 23:30


Mathematics, 12.02.2021 23:30





World Languages, 12.02.2021 23:30

Mathematics, 12.02.2021 23:30




