
Chemistry, 25.03.2021 18:30 Britny2386
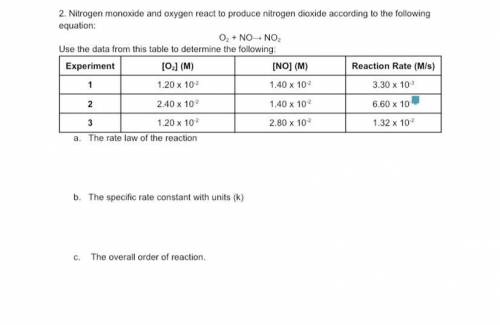
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:
The rate law of the reaction
The specific rate constant with units (k)
The overall order of reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1
You know the right answer?
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:...
Questions


Social Studies, 29.10.2020 21:50

Mathematics, 29.10.2020 21:50

Mathematics, 29.10.2020 21:50

Social Studies, 29.10.2020 21:50


Mathematics, 29.10.2020 21:50


English, 29.10.2020 21:50

History, 29.10.2020 21:50

Mathematics, 29.10.2020 21:50



Mathematics, 29.10.2020 21:50

Mathematics, 29.10.2020 21:50

English, 29.10.2020 21:50


Business, 29.10.2020 21:50

Mathematics, 29.10.2020 21:50



