
Chemistry, 25.03.2021 18:20 phebusadrian01
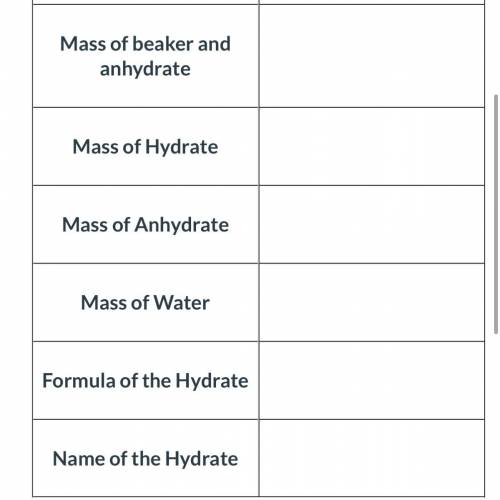
An empty beaker was found to have a mass of 50.49 grams. A hydrate of sodium carbonate was added to the beaker. When the beaker and hydrate was weighed again, the new mass was 62.29 grams. The beaker and the hydrated compound were heated and cooled several times to remove all of the water. The beaker and the anhydrate were then weighed and its new mass was determined to be 59.29 grams.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2


Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
An empty beaker was found to have a mass of 50.49 grams. A hydrate of sodium carbonate was added to...
Questions

Mathematics, 11.03.2022 02:00



Mathematics, 11.03.2022 02:00


English, 11.03.2022 02:00





Biology, 11.03.2022 02:00

Mathematics, 11.03.2022 02:00

Mathematics, 11.03.2022 02:00

Mathematics, 11.03.2022 02:00


History, 11.03.2022 02:00


Mathematics, 11.03.2022 02:00

History, 11.03.2022 02:00



