Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

You know the right answer?
❤️URGENT 10 EACH❤️ty
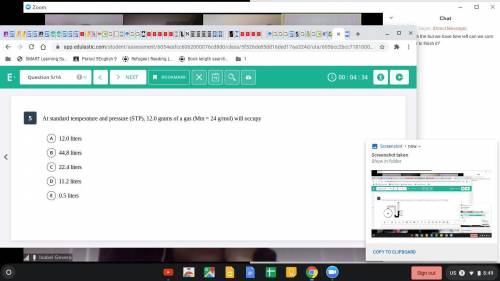
At standard temperature and pressure (STP), 12.0 grams of a gas (Mm=24 g/mol)...
Questions


Mathematics, 10.04.2022 03:40

Chemistry, 10.04.2022 03:50

French, 10.04.2022 04:00



Mathematics, 10.04.2022 04:20

Mathematics, 10.04.2022 04:20


Social Studies, 10.04.2022 04:30



Mathematics, 10.04.2022 04:50






Mathematics, 10.04.2022 05:00