
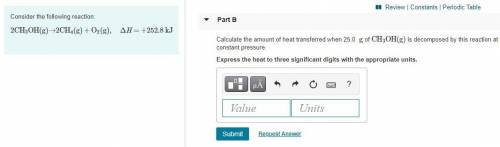
Part B:
Calculate the amount of heat transferred when 25.0 g of CH3OH(g) is decomposed by this reaction at constant pressure. Express the heat to three significant digits with the appropriate units.
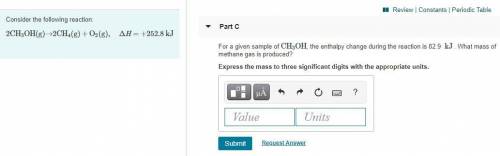
Part C:
For a given sample of CH3OH, the enthalpy change during the reaction is 82.9 kJ. What mass of methane gas is produced? Express the mass to three significant digits with the appropriate units.
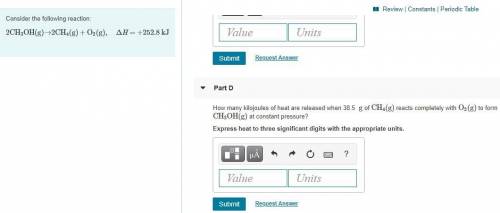
Part D:
How many kilojoules of heat are released when 38.5 g of CH4(g) reacts completely with O2(g) to form CH3OH(g) at constant pressure? Express heat to three significant digits with the appropriate units.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1
You know the right answer?
Part B:
Calculate the amount of heat transferred when 25.0 g of CH3OH(g) is decomposed by this reac...
Questions

Mathematics, 25.01.2020 08:31

Mathematics, 25.01.2020 08:31

Mathematics, 25.01.2020 08:31

Mathematics, 25.01.2020 08:31


Mathematics, 25.01.2020 08:31

Mathematics, 25.01.2020 08:31






Physics, 25.01.2020 08:31





History, 25.01.2020 08:31




