
Chemistry, 25.03.2021 03:20 galaxicorn45
I've been stuck on this
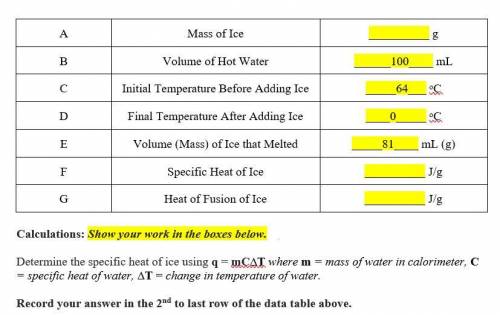
Determine the specific heat of ice using q = mC∆T where m = mass of water in calorimeter, C = specific heat of water, ∆T = change in temperature of water.
Determine the heat of fusion of ice using q = m∆Hf where q = heat, m = mass of substance being melted, ∆Hf = heat of fusion of substance being melted.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2


Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

You know the right answer?
I've been stuck on this
Determine the specific heat of ice using q = mC∆T where m = mass of water i...
Questions

English, 01.09.2019 11:00

Mathematics, 01.09.2019 11:00

Business, 01.09.2019 11:00


Social Studies, 01.09.2019 11:00

English, 01.09.2019 11:00




English, 01.09.2019 11:00

Mathematics, 01.09.2019 11:00


Biology, 01.09.2019 11:00

History, 01.09.2019 11:00




Biology, 01.09.2019 11:00

Social Studies, 01.09.2019 11:00

Mathematics, 01.09.2019 11:00



