PLEASE HELPP!
The proposed mechanism for a reaction is:
Step 1: A + B X (fast)
Step 2:...

Chemistry, 24.03.2021 22:40 nnaaatt1845
PLEASE HELPP!
The proposed mechanism for a reaction is:
Step 1: A + B X (fast)
Step 2: X + C → Y (slow)
Step 3: Y → D (fast)
Which rate law is consistent with this mechanism?
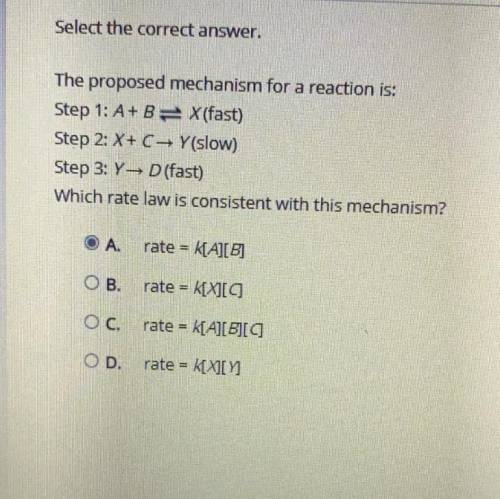

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 14:00
What mass of iron must be in a hot pack to provide -335kj of heat when the iron reacts with oxygen and is converted to iron (111) oxide according to the following thermochemical equation? 2fe(s) + 1.5o2(g)—> fe2o3(s) delta h= -824.2kj/mol
Answers: 1
You know the right answer?
Questions



English, 06.06.2020 21:58





Physics, 06.06.2020 21:58


Mathematics, 06.06.2020 21:58


Mathematics, 06.06.2020 21:58







Mathematics, 06.06.2020 21:58



