
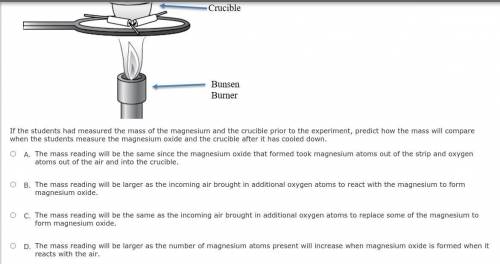
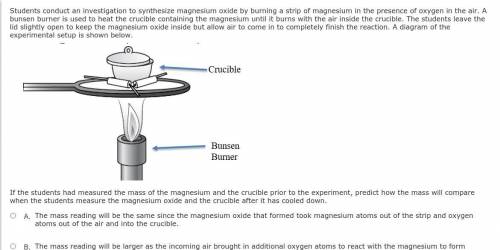
Students conduct an investigation to synthesize magnesium oxide by burning a strip of magnesium in the presence of oxygen in the air. A bunsen burner is used to heat the crucible containing the magnesium until it burns with the air inside the crucible. The students leave the lid slightly open to keep the magnesium oxide inside but allow air to come in to completely finish the reaction. A diagram of the experimental setup is shown below.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Listen base your answer to the question on the information below.propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below.c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol.what is the correct structural formula for a molecule of methanethiol
Answers: 3


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Students conduct an investigation to synthesize magnesium oxide by burning a strip of magnesium in t...
Questions

Mathematics, 10.09.2021 05:40


English, 10.09.2021 05:40

History, 10.09.2021 05:40

Mathematics, 10.09.2021 05:40





History, 10.09.2021 05:40

Geography, 10.09.2021 05:40

Mathematics, 10.09.2021 05:40

Business, 10.09.2021 05:40

Mathematics, 10.09.2021 05:40



History, 10.09.2021 05:40


Mathematics, 10.09.2021 05:40

Advanced Placement (AP), 10.09.2021 05:40



