
Chemistry, 24.03.2021 19:00 kerstynsharp08
How many grams of aluminum oxide would form if 12.5 moles of aluminum burned 4 Al + 3 02 - 2 A1,0,?
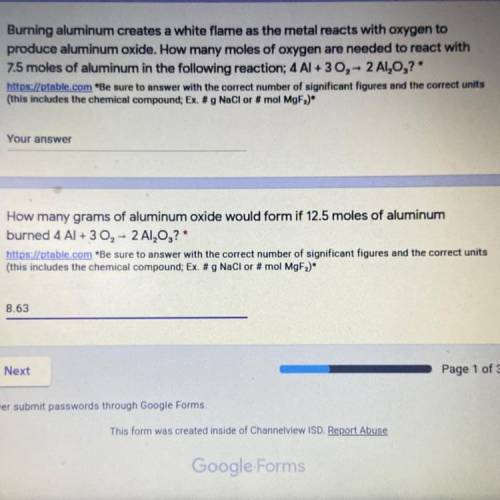

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1


Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
How many grams of aluminum oxide would form if 12.5 moles of aluminum
burned 4 Al + 3 02 - 2 A1,0,?...
Questions


Chemistry, 28.05.2021 02:20




Mathematics, 28.05.2021 02:20


Social Studies, 28.05.2021 02:20


Social Studies, 28.05.2021 02:20










Mathematics, 28.05.2021 02:20



