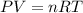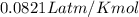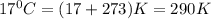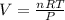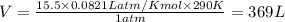Chemistry, 24.03.2021 17:00 mothertrucker2828
1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid dodecane into gaseous carbon dioxide and gaseous water. 2. Suppose 0.22 kg of dodecane are burned in air at a pressure of exactly and a temperature of 17.0°C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
1. Write a balanced chemical equation, including physical state symbols, for the combustion of liqui...
Questions


Mathematics, 01.07.2019 17:30

Mathematics, 01.07.2019 17:30

Social Studies, 01.07.2019 17:30

Computers and Technology, 01.07.2019 17:30


Geography, 01.07.2019 17:30


Mathematics, 01.07.2019 17:30

Biology, 01.07.2019 17:30

Biology, 01.07.2019 17:30

Biology, 01.07.2019 17:30







Biology, 01.07.2019 17:30

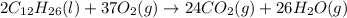
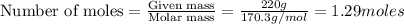
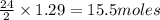 of carbon dioxide
of carbon dioxide