
Chemistry, 24.03.2021 06:10 wittlemarie
Equimolar amounts of Cl2(g) and CO(g) are injected into an evacuated, rigid container, where they react according to the equation below.
Cl2(g) + CO --> COCl2(g)
(a)If 7.0 g of CO(g) is consumed in the reaction with excess Cl2(g), how many moles of COCl2(g) are produced?
(b) Which element is oxidized in this reaction? Justify your answer in terms of oxidation numbers.
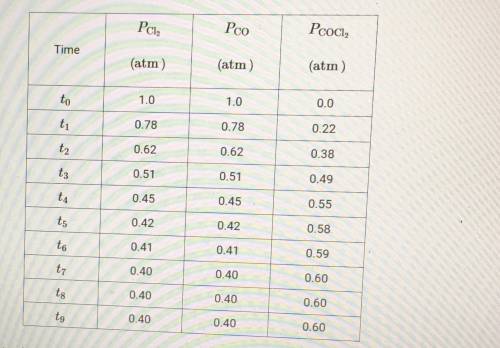
(c) At time t8, is the rate of the forward reaction greater than, less than, or equal to the rate of the reverse reaction? Justify your choice.
(d) At equilibrium, the container holds the most molecules of which gas, Cl2 or COCl2? Explain your answer.
(e) A student hypothesizes that if the temperature of the container is decreased after time t9, the mole fraction of Cl2 in the container will increase. Do you agree or disagree with student's hypothesis? Justify your answer.
(f) Using the data in the table, determine the value of the equilibrium constant, Kp, for the reaction represented by the equation below. Cl2 + C0 <--> COCl2
(g) Using the value determined in part F determine the value of Kp' for the equation shown below. 3Cl2 + 3CO <--> 3COCl2 Kp= ?
(h) Which gas, Cl2 or COCl2, will deviate most from the ideal gas law at low temperature? Justify your choice.
Thanks!!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
Equimolar amounts of Cl2(g) and CO(g) are injected into an evacuated, rigid container, where they re...
Questions








History, 02.07.2019 04:00

Mathematics, 02.07.2019 04:00

Mathematics, 02.07.2019 04:00

Mathematics, 02.07.2019 04:00




Biology, 02.07.2019 04:00

Mathematics, 02.07.2019 04:00


Biology, 02.07.2019 04:00


Mathematics, 02.07.2019 04:00



