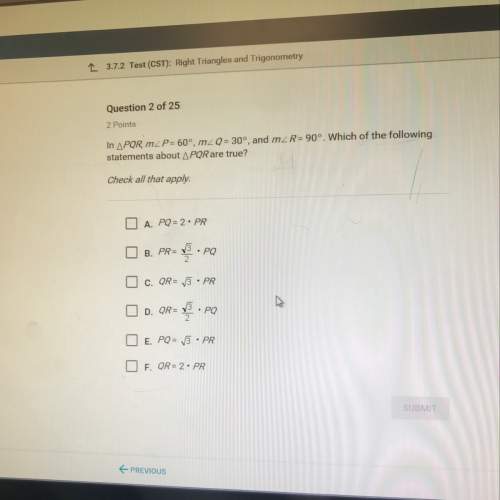
Chemistry, 23.03.2021 20:10 yabfegi9669
2.15(a) A sample consisting of Lo mol of perfect gas molecules with C =
20.87K 'is initially at 3.25 atm and 310 K. It undergoes reversible adiabatic
expansion until its pressure reaches 250 atm. Cakulate the final volume and
temperature and the work done.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
2.15(a) A sample consisting of Lo mol of perfect gas molecules with C =
20.87K 'is initially at 3.2...
Questions

Biology, 05.12.2020 14:00


Health, 05.12.2020 14:00

Mathematics, 05.12.2020 14:00

Mathematics, 05.12.2020 14:00

Mathematics, 05.12.2020 14:00

Mathematics, 05.12.2020 14:00

Computers and Technology, 05.12.2020 14:00

Mathematics, 05.12.2020 14:00



Mathematics, 05.12.2020 14:00




Biology, 05.12.2020 14:00



English, 05.12.2020 14:00

History, 05.12.2020 14:00