125.6 g

Chemistry, 23.03.2021 20:00 armahoney8566
How many grams of water are formed if 42.5g of ammonia (NH3) are oxidized?
10.15 9
125.6 g
12.33 g
67.50 g
(equation in photo)
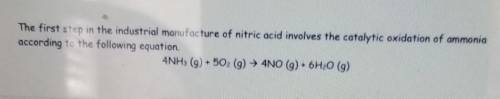

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
How many grams of water are formed if 42.5g of ammonia (NH3) are oxidized?
10.15 9
125.6 g
125.6 g
Questions


English, 14.10.2019 01:50

English, 14.10.2019 01:50

Biology, 14.10.2019 01:50

Mathematics, 14.10.2019 01:50

Mathematics, 14.10.2019 01:50


English, 14.10.2019 01:50

Mathematics, 14.10.2019 01:50


English, 14.10.2019 01:50

History, 14.10.2019 01:50

Mathematics, 14.10.2019 01:50


Mathematics, 14.10.2019 01:50


History, 14.10.2019 01:50





