
Chemistry, 23.03.2021 17:20 LarryJoeseph
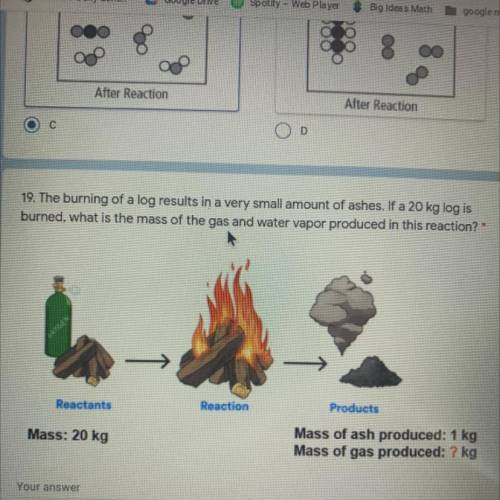
The burning of a log results in a very small amount of ashes. If a 20 kg log is
burned, what is the mass of the gas and water vapor produced in this reaction? *
OXYDEN
Reactants
Reaction
Products
Mass: 20 kg
Mass of ash produced: 1 kg
Mass of gas produced: ? kg

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
The burning of a log results in a very small amount of ashes. If a 20 kg log is
burned, what is the...
Questions


Mathematics, 20.02.2020 19:37


Mathematics, 20.02.2020 19:37










Mathematics, 20.02.2020 19:37








