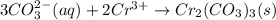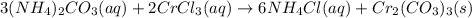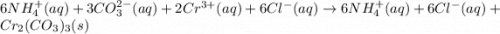Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Consider the reaction when aqueous solutions of ammonium carbonate and chromium(III) chloride are co...
Questions




English, 30.10.2020 20:30

Mathematics, 30.10.2020 20:30







English, 30.10.2020 20:30


Biology, 30.10.2020 20:30




Mathematics, 30.10.2020 20:30

History, 30.10.2020 20:30

Mathematics, 30.10.2020 20:30