
Chemistry, 22.03.2021 14:00 blevinssam274
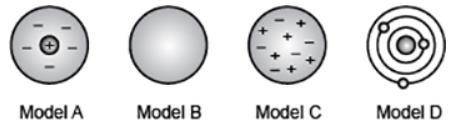
The diagram shows the different models of the atom that eventually led to the modern atomic theory.
Which model was proposed as a result of Rutherford's scattering experiment where positive particles did not pass straight through a foil as expected? (5 points)
Model A
Model B
Model C
Model D

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
The diagram shows the different models of the atom that eventually led to the modern atomic theory....
Questions


Mathematics, 30.10.2020 20:40

Mathematics, 30.10.2020 20:40

English, 30.10.2020 20:40

Health, 30.10.2020 20:40

Mathematics, 30.10.2020 20:40

English, 30.10.2020 20:40





Mathematics, 30.10.2020 20:40

History, 30.10.2020 20:40


Chemistry, 30.10.2020 20:40


Mathematics, 30.10.2020 20:40

Mathematics, 30.10.2020 20:40

Social Studies, 30.10.2020 20:40

History, 30.10.2020 20:40



