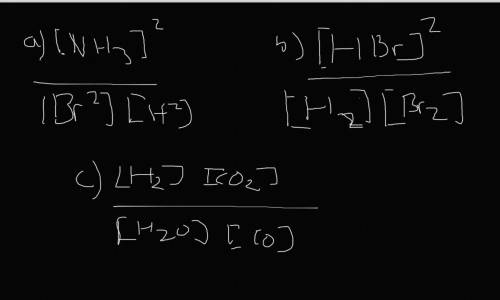Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Write the expressions for the equilibrium constants of the reactions below. (4 points each)
a. N2...
Questions

Geography, 01.01.2020 06:31

History, 01.01.2020 06:31


Mathematics, 01.01.2020 06:31


English, 01.01.2020 06:31


Mathematics, 01.01.2020 06:31


Mathematics, 01.01.2020 06:31

Geography, 01.01.2020 06:31


Biology, 01.01.2020 06:31







Mathematics, 01.01.2020 06:31