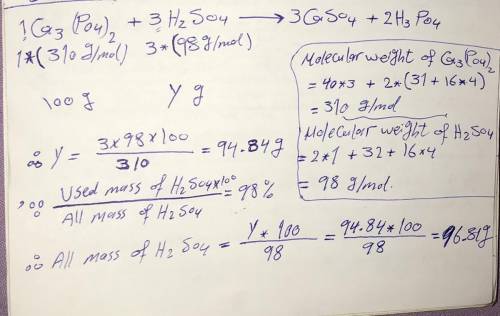Chemistry, 21.03.2021 18:40 kedjenpierrelouis
Ca3(PO4)2 + 3H2SO4 3CaSO4 + 2H3PO4.
What mass of concentrated H2SO4 (98% by mass) must be used to react completely
with 100.00 g of calcium phosphate?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1
You know the right answer?
Ca3(PO4)2 + 3H2SO4 3CaSO4 + 2H3PO4.
What mass of concentrated H2SO4 (98% by mass) must be used to...
Questions

Mathematics, 30.01.2020 04:01




History, 30.01.2020 04:01



Health, 30.01.2020 04:01

Biology, 30.01.2020 04:01

Mathematics, 30.01.2020 04:01

History, 30.01.2020 04:01



Social Studies, 30.01.2020 04:01

Spanish, 30.01.2020 04:01

Mathematics, 30.01.2020 04:01

Mathematics, 30.01.2020 04:01


History, 30.01.2020 04:01