Chemistry, 21.03.2021 06:50 HTKPenguin
Calculate the mass of CO2 that can be produced if the reaction of 54.0 g of propane and sufficient oxygen has a 64.0% yield.
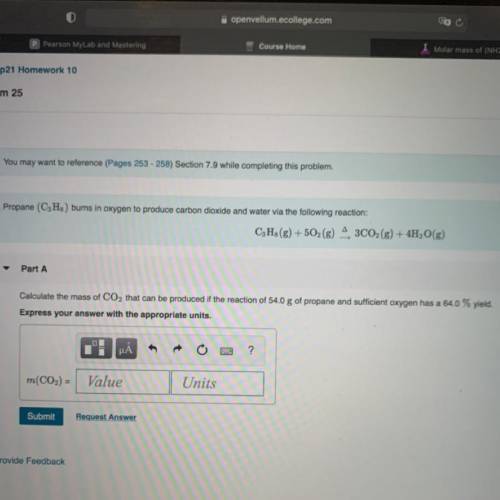

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
Calculate the mass of CO2 that can be produced if the reaction of 54.0 g of propane and sufficient o...
Questions


Mathematics, 18.08.2019 20:30



History, 18.08.2019 20:30


Mathematics, 18.08.2019 20:30

Mathematics, 18.08.2019 20:30


History, 18.08.2019 20:30

Mathematics, 18.08.2019 20:30

Mathematics, 18.08.2019 20:30

Physics, 18.08.2019 20:30


Mathematics, 18.08.2019 20:30

Mathematics, 18.08.2019 20:30

Physics, 18.08.2019 20:30