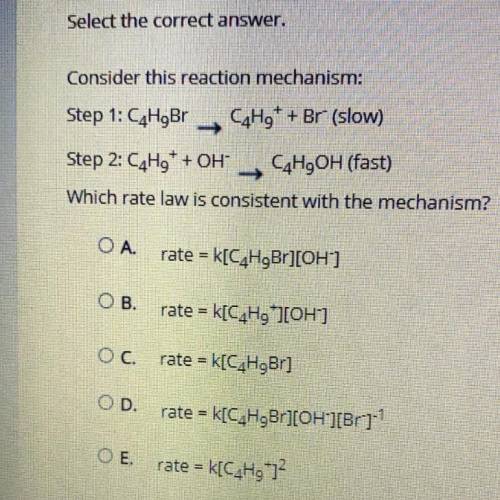
Consider this reaction mechanism:
Step 1: C4H9Br C4H9+ + Br- (slow)
Step 2: C4H9+ + OH- C4H9O...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1
You know the right answer?
Questions



Mathematics, 19.02.2021 20:10

English, 19.02.2021 20:10



English, 19.02.2021 20:10

Geography, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10


Mathematics, 19.02.2021 20:10


Physics, 19.02.2021 20:10



Mathematics, 19.02.2021 20:10



Social Studies, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10