
Chemistry, 19.03.2021 22:20 DaylaReevaFEEVA2757
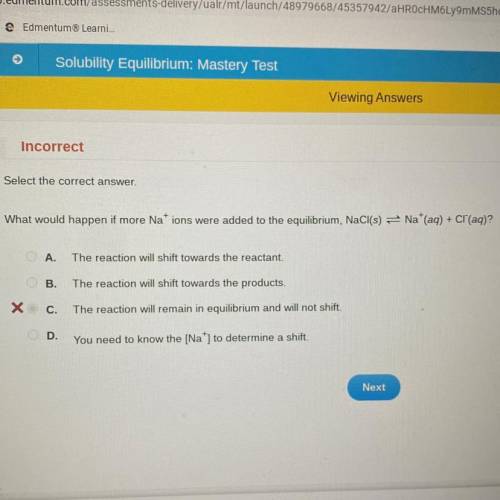
What would happen if more Nations were added to the equilibrium, NaCl(s) = Na+(aq) + Cl(aq)?
A.
The reaction will shift towards the reactant.
B.
The reaction will shift towards the products.
C.
The reaction will remain in equilibrium and will not shift.
OD.
You need to know the [Na] to determine a shift.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

You know the right answer?
What would happen if more Nations were added to the equilibrium, NaCl(s) = Na+(aq) + Cl(aq)?
A.
Questions





English, 20.09.2019 18:30


History, 20.09.2019 18:30











Mathematics, 20.09.2019 18:30

History, 20.09.2019 18:30




