
Chemistry, 19.03.2021 20:30 silverdays566
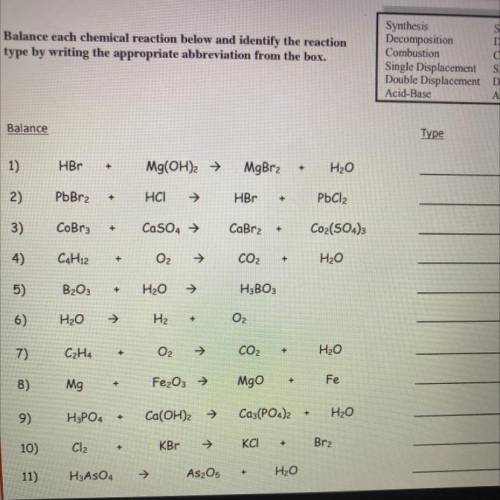
Balance each chemical reaction below and identify the reaction
type by writing the appropriate abbreviation from the box.
Synthesis
SY
Decomposition DC
Combustion
С
Single Displacement SD
Double Displacement DD
Acid-Base
AB
Balance
Type
1)
HBr
+
Mg(OH)2 →
MgBr2
+
H2O
2)
PbBr2
+
HCI
HBr
+
PbCl2
3)
CoBru
+
CaSO4 →
CaBr2
+
Coz(504)3
4)
C4H12
+
O2
CO2
+
H2O
5)
B2O3
+
H2O
H3BO3
6)
H₂O
H₂
+
O2
7)
C₂H4
+
O2
CO2
+
H2O
8)
+
Mg
+
Fe₂O₃ →
Mgo
Fe
9)
+
H3PO4
+
Ca(OH)2 →
Ca3(PO4)2
H2O
10)
Cl2
+
KBT
+
KCI
Brz
11)
+
H3ASO4
As2O5
H₂O

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Balance each chemical reaction below and identify the reaction
type by writing the appropriate abbr...
Questions



Mathematics, 06.05.2020 00:40


English, 06.05.2020 00:40

History, 06.05.2020 00:40

Chemistry, 06.05.2020 00:40


English, 06.05.2020 00:40

History, 06.05.2020 00:40


History, 06.05.2020 00:40

Mathematics, 06.05.2020 00:40






Mathematics, 06.05.2020 00:40

Mathematics, 06.05.2020 00:40



