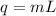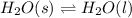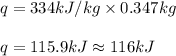Chemistry, 15.12.2019 14:31 kedjenpierrelouis
What is the total amount of heat required to completely melt 347 grams of ice at its melting point?
1)334 j
2) 1450 j
3) 116,000 j
4) 784,000 j

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
What is the total amount of heat required to completely melt 347 grams of ice at its melting point?...
Questions

English, 30.06.2019 05:00


History, 30.06.2019 05:00


History, 30.06.2019 05:00


Mathematics, 30.06.2019 05:00

English, 30.06.2019 05:00


History, 30.06.2019 05:00

Mathematics, 30.06.2019 05:00

History, 30.06.2019 05:00

Mathematics, 30.06.2019 05:00

Biology, 30.06.2019 05:00




English, 30.06.2019 05:00


Mathematics, 30.06.2019 05:00