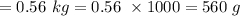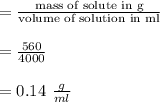Chemistry, 19.03.2021 18:30 thutch1950oww9q0
A chemistry student is given 4.00 L of a clear aqueous solution at 22°C. He is told an unknown amount of a certain compound X is dissolved in the solution. The student allows the solution to cool to 22 C. The solution remains clear. He then evaporates all of the water under vacuum. A precipitate remains. The student washes, dries and weighs the precipitate. It weighs 0.56 kg.
Required:
Using only the information above, Calculate the solubility of X in water at 22° C. If you said yes, calculate it Be sure your answer has a unit symbol and 2 no .0 it. significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
A chemistry student is given 4.00 L of a clear aqueous solution at 22°C. He is told an unknown amou...
Questions

SAT, 23.12.2020 19:30


Computers and Technology, 23.12.2020 19:30

Social Studies, 23.12.2020 19:30

Mathematics, 23.12.2020 19:30

Social Studies, 23.12.2020 19:30

Physics, 23.12.2020 19:30



Advanced Placement (AP), 23.12.2020 19:30

English, 23.12.2020 19:30


Social Studies, 23.12.2020 19:30

Social Studies, 23.12.2020 19:30



Mathematics, 23.12.2020 19:30

English, 23.12.2020 19:30

Mathematics, 23.12.2020 19:30