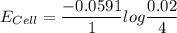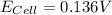Chemistry, 19.03.2021 18:20 sunshine52577oyeor9
A certain metal M forms a soluble nitrate salt M(NO3), Suppose the left half cell ofa galvanic cell apparatus is filled with a 4.00 M solution of M (NO,), and the right half cell with a 20.0 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0 °C.
Required:
a. Which electrode will be positive?
b. What voltage will the voltmeter show?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
A certain metal M forms a soluble nitrate salt M(NO3), Suppose the left half cell ofa galvanic cell...
Questions

English, 02.12.2021 14:00

English, 02.12.2021 14:00

Advanced Placement (AP), 02.12.2021 14:00

English, 02.12.2021 14:00


Physics, 02.12.2021 14:00

Geography, 02.12.2021 14:00


Chemistry, 02.12.2021 14:00

English, 02.12.2021 14:00


Chemistry, 02.12.2021 14:00

English, 02.12.2021 14:00

Mathematics, 02.12.2021 14:00



History, 02.12.2021 14:00

Mathematics, 02.12.2021 14:00

English, 02.12.2021 14:00