
Chemistry, 19.03.2021 06:30 Sanchezj104
2 CH3OH + 3 02 2 CO2 + 4H2O
What is the mass of oxygen (O2) that is required to produce 579 g of carbon dioxide (CO2)?
. Your answer should have three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
2 CH3OH + 3 02 2 CO2 + 4H2O
What is the mass of oxygen (O2) that is required to produce 579 g of ca...
Questions



Computers and Technology, 16.10.2019 03:30














Mathematics, 16.10.2019 03:30

English, 16.10.2019 03:30


Spanish, 16.10.2019 03:30

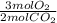 = 19.74 mol O₂
= 19.74 mol O₂

