
Chemistry, 19.03.2021 02:40 cheefdogg420
Liquids B and C are partially miscible at 25 oC. When one starts with 1 mol of C at 25 oC and isothermally adds B a little at a time, a two-phase system first appears when a bit more than 0.125 mol of B has been added; continuing to add B, one finds the two-phase system becomes a one-phase system when a total of 3 mol of B has been added. For a system consisting of 2.5 mol of B and 2 mol of C at 25 oC, find the number of moles of B and of C present in each phase.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
You know the right answer?
Liquids B and C are partially miscible at 25 oC. When one starts with 1 mol of C at 25 oC and isothe...
Questions


Mathematics, 19.07.2019 22:00

History, 19.07.2019 22:00






History, 19.07.2019 22:00

English, 19.07.2019 22:00

Social Studies, 19.07.2019 22:00

Mathematics, 19.07.2019 22:00

History, 19.07.2019 22:00




Biology, 19.07.2019 22:00


Mathematics, 19.07.2019 22:00

Biology, 19.07.2019 22:00

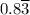 moles of A and 2.5 moles of B in the one-phase system
moles of A and 2.5 moles of B in the one-phase system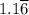 moles of A and
moles of A and 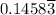 moles of B in the two-phase system
moles of B in the two-phase system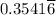 moles of B remains in the system
moles of B remains in the system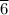 moles of A
moles of A

