
Chemistry, 18.03.2021 23:40 larueeee25
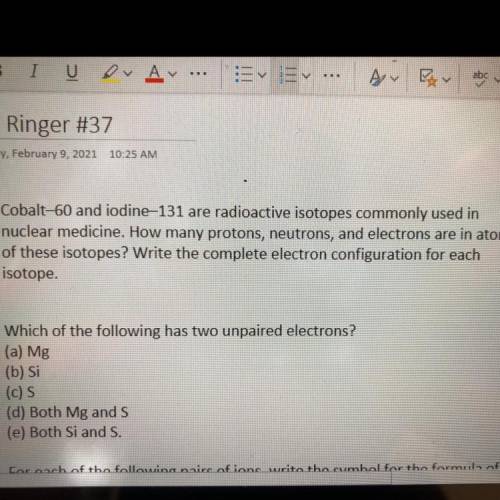
Cobalt-60 and iodine 131 are radioactive isotopes commonly used in
nuclear medicine. How many protons, neutrons, and electrons are in atoms
of these isotopes? Write the complete electron configuration for each
isotope.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
Cobalt-60 and iodine 131 are radioactive isotopes commonly used in
nuclear medicine. How many proto...
Questions

Mathematics, 06.04.2021 21:40




Mathematics, 06.04.2021 21:40



History, 06.04.2021 21:40

History, 06.04.2021 21:40



Mathematics, 06.04.2021 21:40

Mathematics, 06.04.2021 21:40






Computers and Technology, 06.04.2021 21:40



