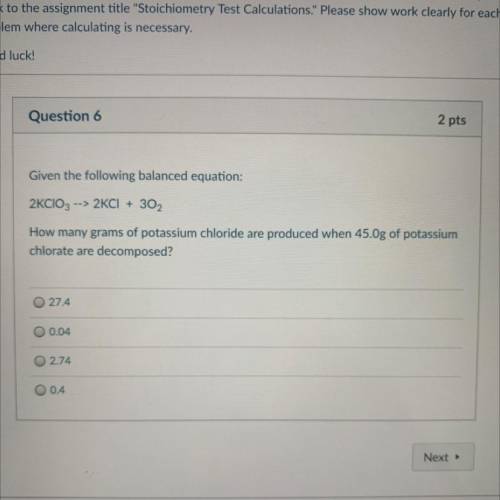
Given the following balanced equation:
2KCIO3 --> 2KCI + 302
How many grams of potassium c...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Questions


Mathematics, 29.06.2019 18:30

Mathematics, 29.06.2019 18:30

English, 29.06.2019 18:30


Spanish, 29.06.2019 18:30

Mathematics, 29.06.2019 18:30

Mathematics, 29.06.2019 18:30

Mathematics, 29.06.2019 18:30

Spanish, 29.06.2019 18:30

Business, 29.06.2019 18:30

Mathematics, 29.06.2019 18:30



World Languages, 29.06.2019 18:30




Mathematics, 29.06.2019 18:30