
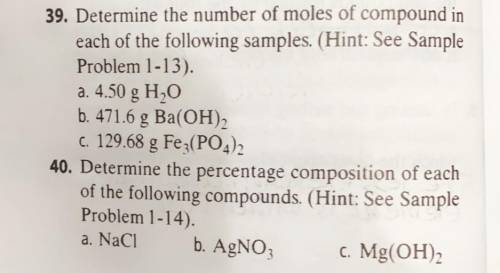
39. Determine the number of moles of compound in
each of the following samples. (Hint: See Sample
Problem 1-13).
a. 4.50 g H2O
b. 471.6 g Ba(OH)2
C. 129.68 g Fe3(PO4)2
40. Determine the percentage composition of each
of the following compounds. (Hint: See Sample
Problem 1-14).
b. AgNO3
.c. Mg(OH)2
a. NaCl
Help me please for this two question

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
39. Determine the number of moles of compound in
each of the following samples. (Hint: See Sample
Questions



English, 27.06.2019 20:30


English, 27.06.2019 20:30

Arts, 27.06.2019 20:30

Mathematics, 27.06.2019 20:30


Social Studies, 27.06.2019 20:30

Physics, 27.06.2019 20:30

Mathematics, 27.06.2019 20:30

English, 27.06.2019 20:30

History, 27.06.2019 20:30

Mathematics, 27.06.2019 20:30


Mathematics, 27.06.2019 20:30






