
Chemistry, 18.03.2021 09:20 queenkimm26
C.
A 35.5 g cube of aluminum initially at 48.5° C is submerged into 105.3 g of water at 15.4
What is the final temperature of both substances at thermal equilibrium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
C.
A 35.5 g cube of aluminum initially at 48.5° C is submerged into 105.3 g of water at 15.4
...
...
Questions



Social Studies, 19.01.2021 21:50


Mathematics, 19.01.2021 21:50

Mathematics, 19.01.2021 21:50

English, 19.01.2021 21:50


English, 19.01.2021 21:50

Social Studies, 19.01.2021 21:50

Physics, 19.01.2021 21:50



History, 19.01.2021 21:50

Spanish, 19.01.2021 21:50

Mathematics, 19.01.2021 21:50



Chemistry, 19.01.2021 21:50

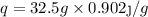 °
°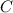
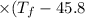 °
°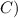
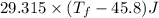
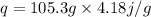 °
°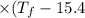 °
°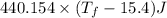
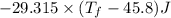
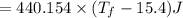
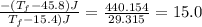
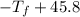 °
°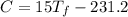 °
°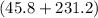 °
°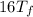
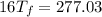 °
°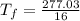 = 17.3°C
= 17.3°C

