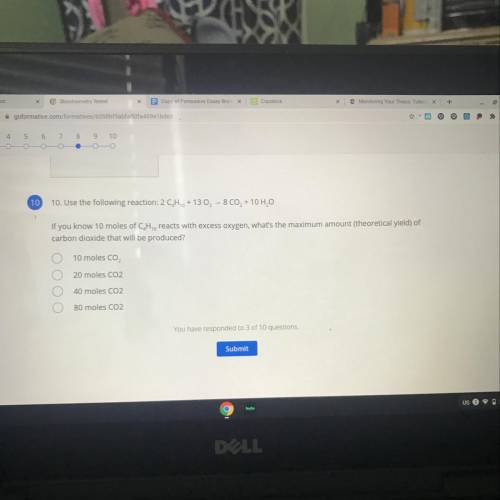
Use the following reaction:
2 CH2 + 13 O2 - 8 CO2 + 10 H2O
If you know 10 moles of C, H, reac...

Chemistry, 18.03.2021 03:30 anonymousanon
Use the following reaction:
2 CH2 + 13 O2 - 8 CO2 + 10 H2O
If you know 10 moles of C, H, reacts with excess oxygen, what's the maximum amount (theoretical yield) of
carbon dioxide that will be produced?
A. 10 moles CO,
B. 20 moles CO2
C. 40 moles CO2
D. 80 moles CO2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Questions



Mathematics, 01.10.2021 16:50

Mathematics, 01.10.2021 16:50

Chemistry, 01.10.2021 16:50


Chemistry, 01.10.2021 17:00


English, 01.10.2021 17:00


Law, 01.10.2021 17:00

Mathematics, 01.10.2021 17:00


Mathematics, 01.10.2021 17:00

Mathematics, 01.10.2021 17:00




Chemistry, 01.10.2021 17:00



