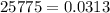Chemistry, 18.03.2021 03:10 hiiamsuperverylong
Methane gas is collected over water at 25 C and an atmospheric pressure of 775 mmHg. What is the dry pressure of the gas at this temperature? (Vapor Pressure of water = 0.0313 atm).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
Methane gas is collected over water at 25 C and an atmospheric pressure of 775 mmHg. What is the dry...
Questions








English, 14.04.2021 14:00

Chemistry, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00


Spanish, 14.04.2021 14:00


Chemistry, 14.04.2021 14:00


Mathematics, 14.04.2021 14:00


Mathematics, 14.04.2021 14:00