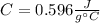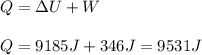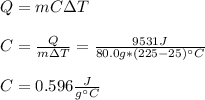Chemistry, 18.03.2021 02:50 genyjoannerubiera
An 80.0 g sample of a gas was heated from 25 °C to 225 °C. During this process, 346 J of work was done by the system and its internal energy increased by 9185 J. What is the specific heat of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
An 80.0 g sample of a gas was heated from 25 °C to 225 °C. During this process, 346 J of work was do...
Questions

Mathematics, 16.10.2019 12:10


Chemistry, 16.10.2019 12:10


Biology, 16.10.2019 12:10

History, 16.10.2019 12:10

History, 16.10.2019 12:10



Computers and Technology, 16.10.2019 12:10

Mathematics, 16.10.2019 12:10

Mathematics, 16.10.2019 12:20


Arts, 16.10.2019 12:20

Social Studies, 16.10.2019 12:20



Physics, 16.10.2019 12:20