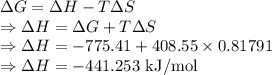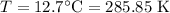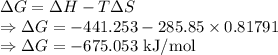Chemistry, 18.03.2021 02:50 Mw3spartan17
For a particular reaction at 135.4 °C, Δ=−775.41 kJ/mol, and Δ=817.91 J/(mol⋅K).
Calculate ΔG for this reaction at 12.7 °C.
Δ=

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
For a particular reaction at 135.4 °C, Δ=−775.41 kJ/mol, and Δ=817.91 J/(mol⋅K).
Calculate ΔG for t...
Questions

Mathematics, 18.02.2021 23:40


Mathematics, 18.02.2021 23:40



Mathematics, 18.02.2021 23:40



Mathematics, 18.02.2021 23:40



Mathematics, 18.02.2021 23:40

Mathematics, 18.02.2021 23:40

History, 18.02.2021 23:40

Mathematics, 18.02.2021 23:40

History, 18.02.2021 23:40

Mathematics, 18.02.2021 23:40

Chemistry, 18.02.2021 23:40


Social Studies, 18.02.2021 23:40

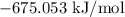
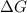 = Gibbs free energy =
= Gibbs free energy = 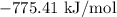
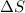 = Change in entropy =
= Change in entropy = 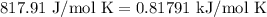
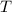 = Temperature =
= Temperature = 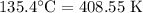
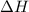 = Change in enthalpy
= Change in enthalpy