
Chemistry, 18.03.2021 02:40 HalpMahOnMahH0meW0rk
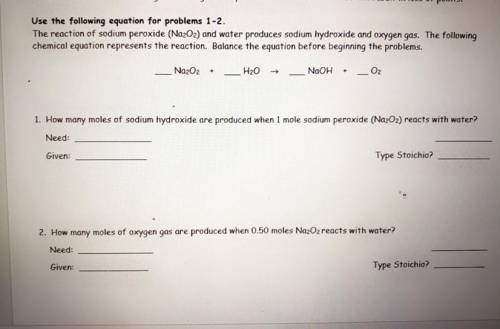
the reaction of sodium peroxide (Na2O2) and water produces sodium hydroxide and oxygen gas. the following chemical equation represents the reaction. balance the equation before beginning the problems. ( use the equation for problems one and two )

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1


Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2
You know the right answer?
the reaction of sodium peroxide (Na2O2) and water produces sodium hydroxide and oxygen gas. the foll...
Questions

Chemistry, 25.11.2021 16:20


Social Studies, 25.11.2021 16:20




Spanish, 25.11.2021 16:20


English, 25.11.2021 16:20


English, 25.11.2021 16:20

Computers and Technology, 25.11.2021 16:20


Physics, 25.11.2021 16:30

History, 25.11.2021 16:30



Mathematics, 25.11.2021 16:30

Mathematics, 25.11.2021 16:30

Mathematics, 25.11.2021 16:30



