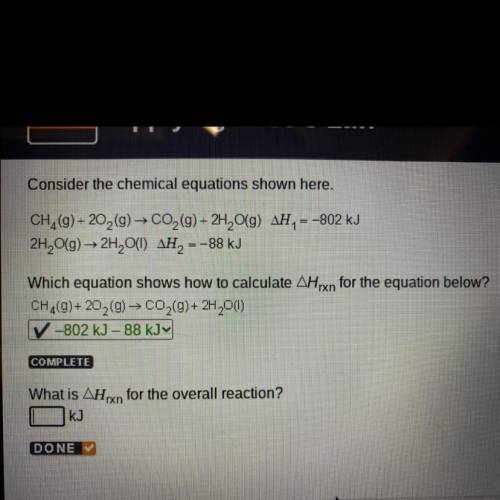
Consider the chemical equations shown here.
CH (9) +20,(9) → CO2 (9)+ 2H, O(9) AH, = -802 kJ
...

Chemistry, 18.03.2021 02:40 graceduke2005p6z8yp
Consider the chemical equations shown here.
CH (9) +20,(9) → CO2 (9)+ 2H, O(9) AH, = -802 kJ
2H2O(g) → 2H, O(1) AH, -
= -88 kJ
Which equation shows how to calculate Arxn for the equation below?
CH,(9) + 20 (0) - CO,(9)+ 2H 0(1)
-802 kJ - 88 KJY
COMPLETE
What is AHxn for the overall reaction?
KJ
PLEASE FOCUS ON THE SECOND PART !!
Help

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3


You know the right answer?
Questions

Mathematics, 18.11.2020 04:10




Mathematics, 18.11.2020 04:10



Computers and Technology, 18.11.2020 04:10

Mathematics, 18.11.2020 04:10


History, 18.11.2020 04:10




History, 18.11.2020 04:10




Chemistry, 18.11.2020 04:10

Spanish, 18.11.2020 04:10



