
Chemistry, 18.03.2021 02:30 gildedav001
PLS HELP ASAP!! 50 PTS + BRAINLIEST
Calculate the equilibrium constant, Keq, using the balanced equation, given that the reaction occurs at 25C.
The concentration are as follows: SO2 = 0.90M, O2 = 0.35M, and SO3 = 1.4M
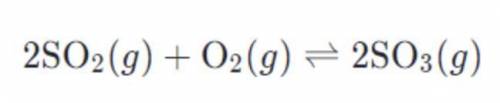

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
PLS HELP ASAP!! 50 PTS + BRAINLIEST
Calculate the equilibrium constant, Keq, using the balanced equ...
Questions


Mathematics, 02.04.2021 07:10

Mathematics, 02.04.2021 07:10


Arts, 02.04.2021 07:10


Social Studies, 02.04.2021 07:10

Mathematics, 02.04.2021 07:10


History, 02.04.2021 07:10

Physics, 02.04.2021 07:10

Chemistry, 02.04.2021 07:20


Mathematics, 02.04.2021 07:20



French, 02.04.2021 07:20


Mathematics, 02.04.2021 07:20



